Foundation Medicine
Redesigned a critical reporting system into a streamlined tool.
What I did
- User research
- User journey mapping
- Product roadmap
- Designed new product features
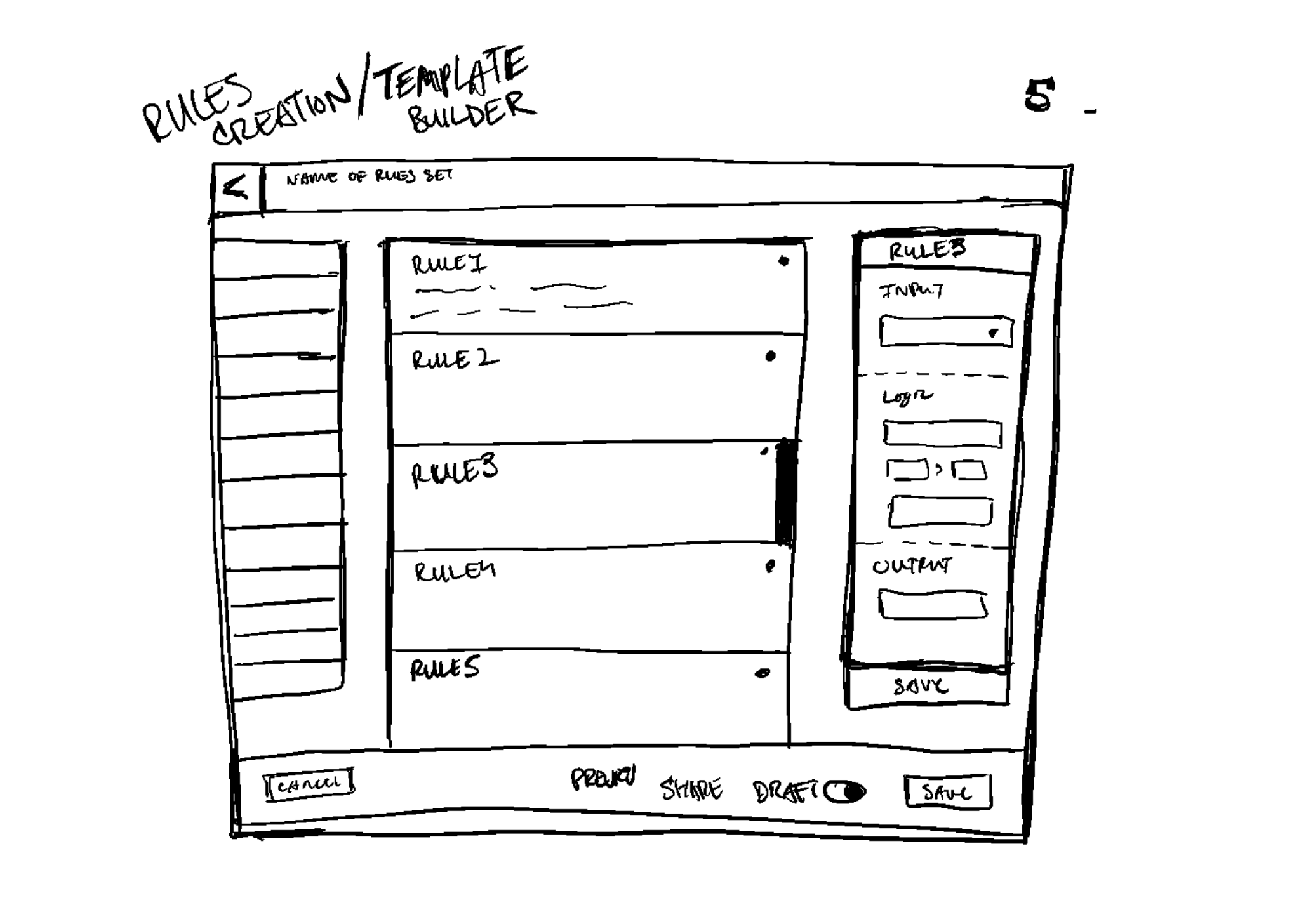
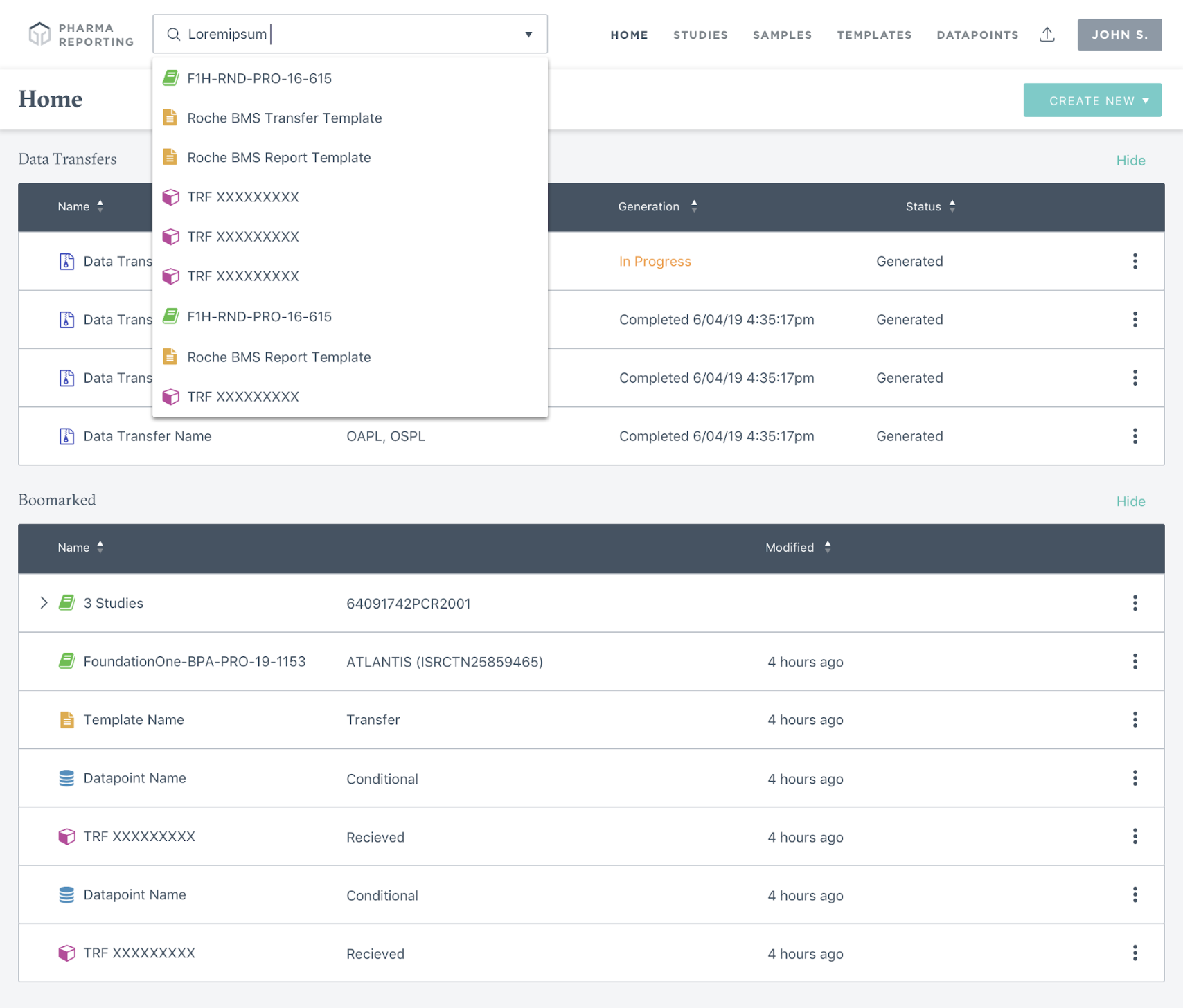
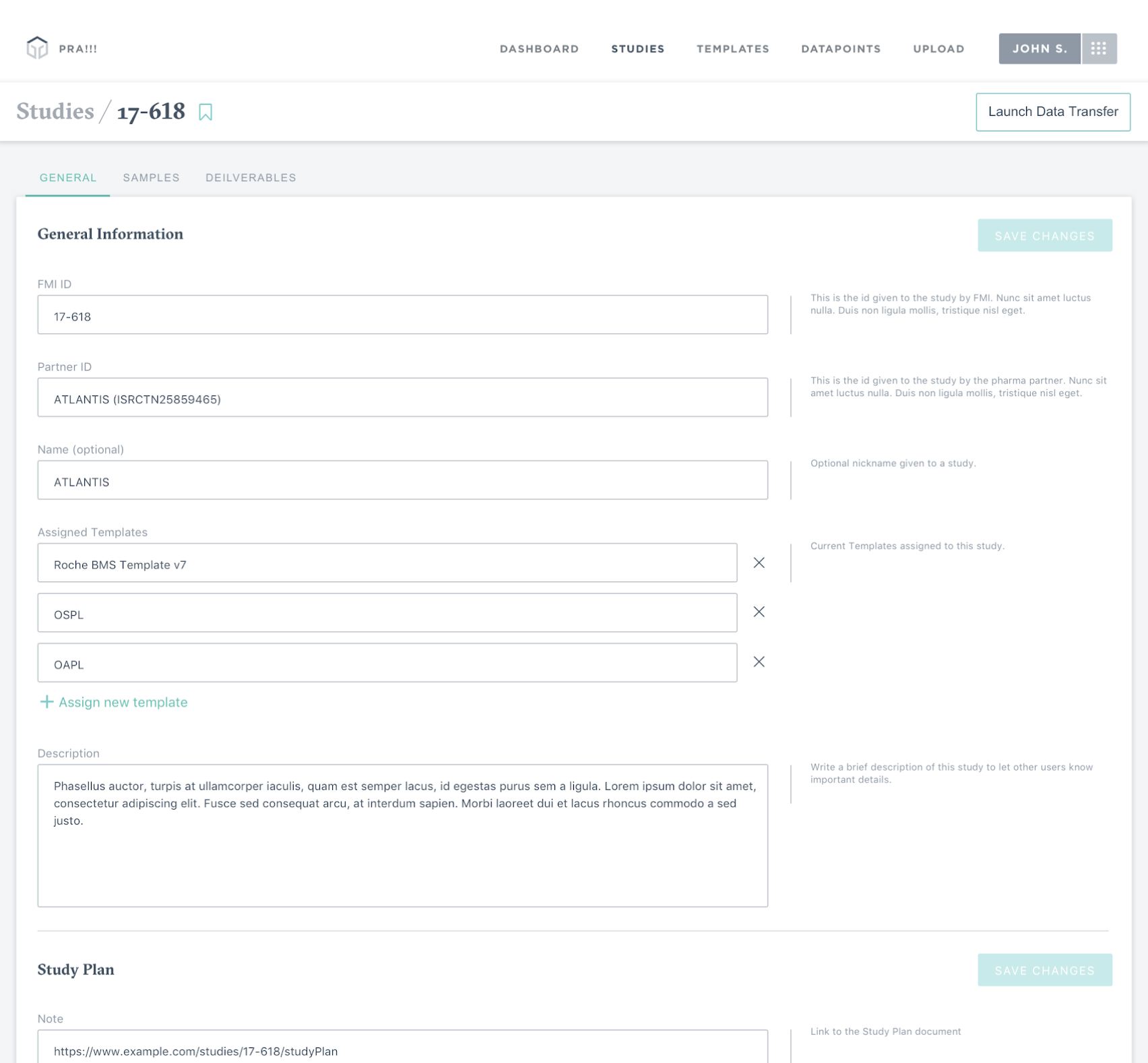
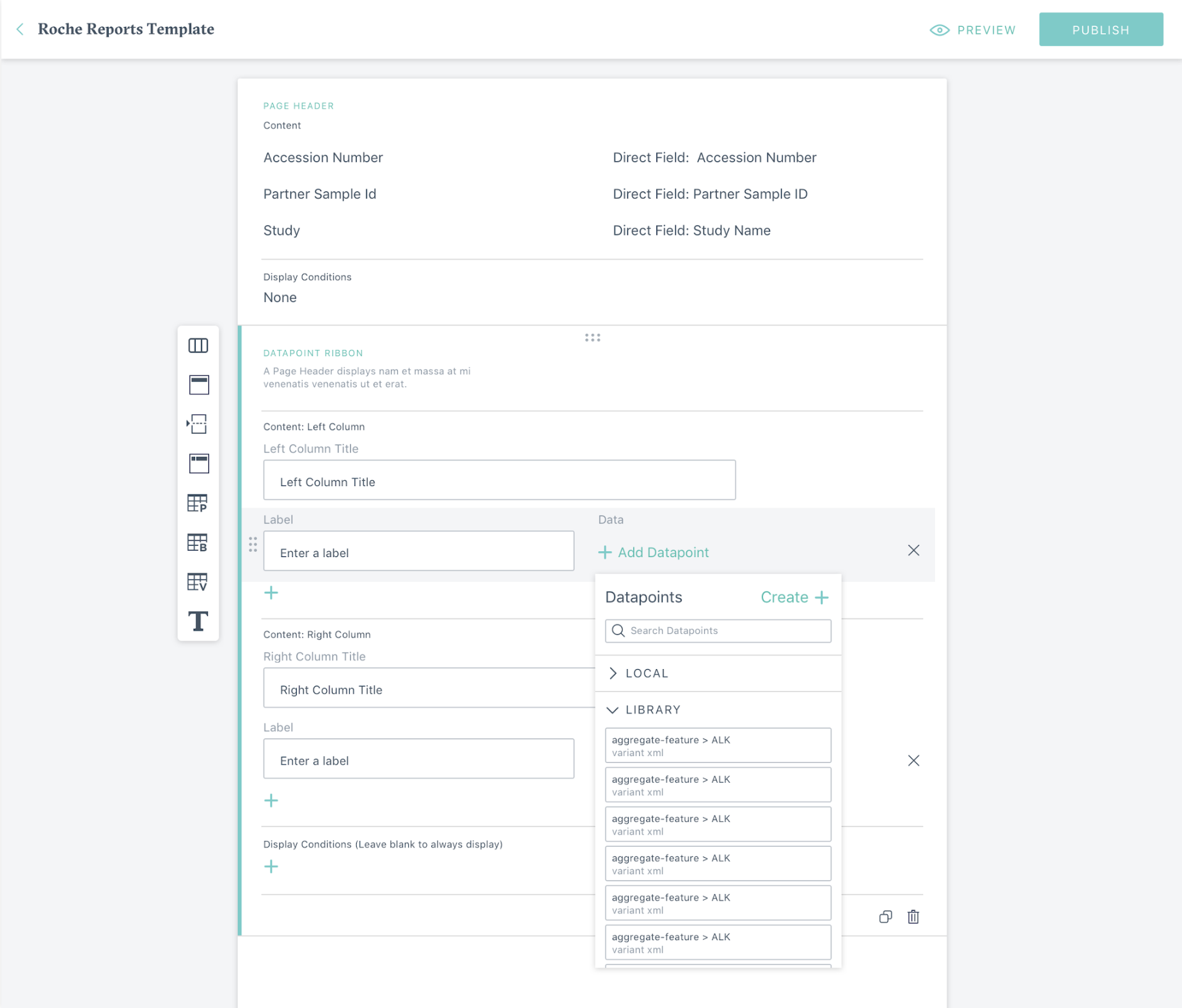
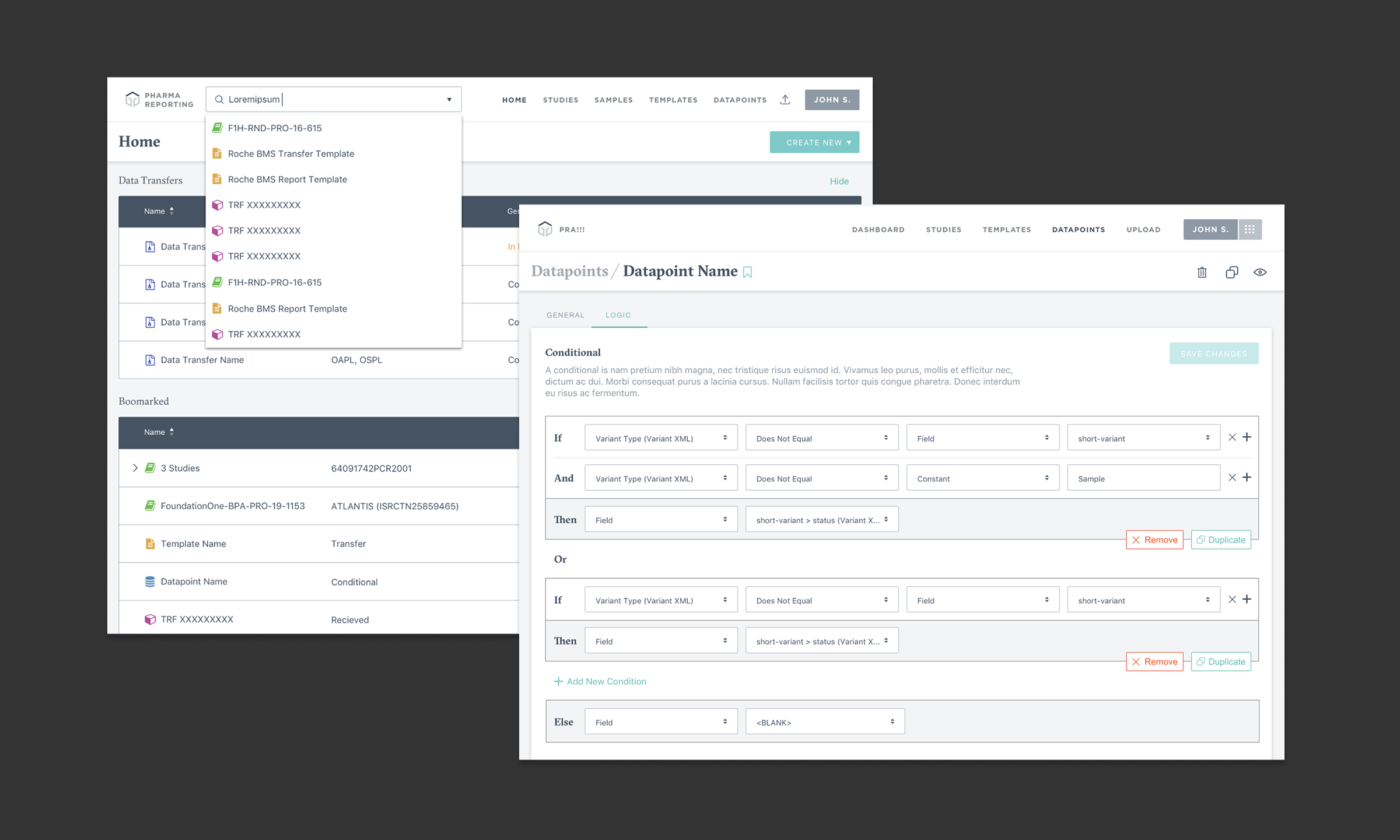
Project Overview
The Pharma Reporting Application (PRA) is a critical tool that enables pharmaceutical partners to qualify patients for clinical trials and validate research findings. However, the existing system had become a bottleneck, with inconsistent functionality and interface issues forcing the Data Operations team to spend more time troubleshooting than executing.
Given the directive to "get PRA out of the way," I led a comprehensive discovery and design initiative to reimagine the application from the ground up. We mapped out ideal user journeys for both external and internal users, established a prioritized roadmap for features, and designed and implemented new features. We were able to transform PRA into a proactive system that increases throughput, reduces errors, and creates an intuitive user experience for pharmaceutical research operations.